Newly discovered glutamate transporter’s elevator-like structure and dual-function mechanism open up a field of possibilities
To maintain normal brain function, the extracellular levels of necessary neurotransmitters, such as glutamate—a major chemical signal responsible for communication between brain cells– have to be kept low to avoid excessive stimulation of receptors and nerve cell damage, a pathological process otherwise referred to as excitotoxicity.
Using a cool twisting, elevator-like mechanism inside the cell membrane, glutamate transporters mediate clearance of glutamate from the synapses between neurons, thereby ensuring that our brain cells are “talking” to each other effectively and safely. But that is not the only role played by these transporters, as they seem to switch to a hidden secondary mode of action right in the middle of their main transporter function.
A research study, led by an international team of scientists, including Center for Biophysics and Quantitative Biology Affiliate Professor Emad Tajkhorshid and doctoral student Shashank Pant, titled “Glutamate transporters contain a conserved chloride channel with two hydrophobic gates,” unveiled the exquisite structure of the glutamate transporter, in addition to the multiple tasks assumed by this molecular machine.
According to Professor Tajkhorshid, membrane transporters are famous for their reliance on “molecular dancing” to accomplish their function, and it is not too surprising that during this dance they may form structures with completely different functions, though in a transient manner. “Here we devise a plan employing a concerted set of computational and experimental techniques to trap such intermediate states for glutamate transporters and to structurally and functionally characterize how they work as ion channels.”
“The work highlights the power of synergistic interaction of computational microscopy, providing the most detailed picture of how proteins function, and experimental structural biology, providing realistic views into the protein structure,” added Tajkhorshid.
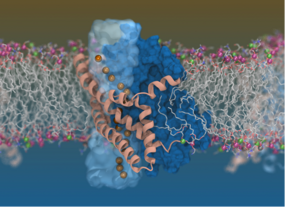
“This research started with a basic question: ‘How do brain cells talk to each other?’” said Pant. “To address this important question, which has direct implications in various neurological disorders, including Alzheimer’s disease, we exploited the power of concerted structural biology and computational biophysics in a very important class of membrane proteins involved in the neurotransmission of the brain.”
“We unraveled the alter ego of a transporter, depicting how it magically develops an ion channel property right in the middle of its normal cycle. This involved a beautiful combination of experimental structural work and computational biophysics,” said Pant.
The findings of this research have been published in Nature.
The research team employed molecular simulations in combination with cryogenic electron microscopy (cryo-EM) to capture for the first time the mechanism of elevator-like glutamate transporters in detail. Cryo-EM allows for a clear depiction of the workings of proteins that cannot be easily examined by other imaging techniques.
This breakthrough discovery led researchers to observe that the transporters not only move chemicals across the cell membrane, but also conduct water and ions simultaneously.
This revelation is expected to pave the way for further investigations targeting the potential causes of several neurological diseases. According to Pant, “this is the first ever structure of this dual-function molecular machine to be observed in the open chloride conducting state, and we believe the findings of this study could have significant impact on future drug design, specifically in the area of neurological diseases.”
In addition to Professor Emad Tajkhorshid and Shashank Pant (PhD student) of the University of Illinois at Urbana-Champaign, the research group leading this study include Ichia Chen (PhD Student), Professor Renae Ryan, Professor Robert Vandenberg, Dr Josep Font, Dr Qianyi Wu from the School of Medical Sciences, Faculty of Medicine and Health, University of Sydney; Alastair Stewart and Dr Meghna Sobti from The Victor Chang Cardiac Research Institute, UNSW; and Dr Rosemary Cater from the Department of Physiology and Cellular Biophysics, Columbia University Irving Medical Center, New York.
Published March 18, 2021 15:49